The structure of vitamin C is shown below. Which one of the following statements concerning this structure is not correct? 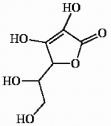
A) The molecule contains 2 pi bonds.
B) The molecule contains sp3 hybridized oxygen atoms.
C) The molecule contains 3 sp2 hybridized carbon atoms.
D) The molecule can be classified as an aldehyde.
E) The molecule contains more than one hydroxyl group.
Correct Answer:
Verified
Q41: The CCN bond angle in acrylonitrile (CH2=CHCN)
Q42: Choose the correct hybridization for the atom
Q48: How many s bonds does the compound
Q48: The CCO bond angle in acetone (CH3COCH3)
Q49: The molecule shown below contains _ pi
Q50: What is the hybridization of the nitrogen
Q55: Acrylonitrile (CH2=CHCN) contains _ s bonds and
Q56: Triethylamine [(CH3CH2)3N] is a molecule in which
Q56: Which of the following statements concerning the
Q59: The HCN bond angle in hydrogen cyanide
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents