Which of the following best describes the relationship between the two structures shown? 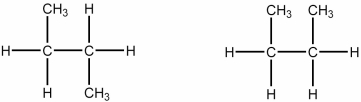
A) They represent the same compound.
B) They represent different compounds that are constitutional isomers.
C) They represent different compounds that are geometric isomers.
D) They represent different compounds that are alkenes.
E) They represent different compounds that are alkanes.
Correct Answer:
Verified
Q65: Boron trifluoride (BF3) is a molecule in
Q66: Structures which differ only in rotations about
Q70: Are the two compounds shown below best
Q70: Provide the skeletal structures of the five
Q71: Are the two compounds shown below
Q72: Explain why the free rotation about the
Q73: The molecule shown below contains _ sigma
Q76: Are the two compounds shown below best
Q77: Are the two compounds shown below
Q78: Provide the hybridization of oxygen in dimethyl
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents