Add six pi bonds to the structure below to produce the most stable isomer. 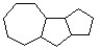
Correct Answer:
Verified
Q43: Classify cyclopentadienyl cation as aromatic, antiaromatic, or
Q44: Classify cyclopropenyl cation as aromatic, antiaromatic, or
Q46: When cycloheptatriene is deprotonated, an anion with
Q46: Rank the following in order of increasing
Q47: Is the molecule below aromatic, antiaromatic, or
Q48: Why would the reaction below proceed at
Q49: Classify the compound below as aromatic, antiaromatic,
Q50: Nitrogen's lone pair electrons occupy what type
Q52: Which sequence ranks the indicated protons in
Q53: Which of the labeled H atoms (1
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents