When 1 mole of anhydrous HCl is reacted with excess 1,3-pentadiene, both the 1,2 and the 1,4-addition products are formed. Which of the following structures shown below is the least likely to be one of these products? (Note: When a chiral carbon is formed in this reaction a racemic mixture results, only one of the two possible enantiomers is shown.)
A) 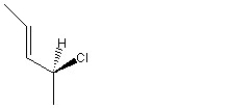
B) 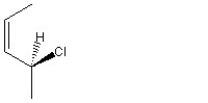
C) 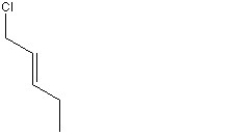
D) 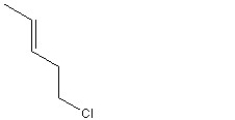
E) 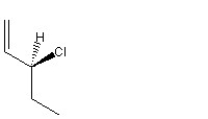
Correct Answer:
Verified
Q30: When (S)-3-bromopent-1-ene is heated in water, which
Q31: Name the two major products which are
Q32: When 1-methylcyclohex-2-en-1-ol is treated with HBr, two
Q34: Draw structures for the two major products
Q34: Draw the LUMO of 1,3-butadiene.
Q36: Provide the two major organic products of
Q39: Give a representation of the lowest occupied
Q39: Provide a detailed, stepwise mechanism for the
Q40: Draw the HOMO of 1,3-butadiene.
Q40: Provide the two major organic products of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents