One or more of the atoms in the structure shown should have nonzero formal charges. Redraw the structure and indicate any such charges. 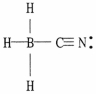
Correct Answer:
Verified
Q25: The formal charge on oxygen in dimethyl
Q32: Draw a correct Lewis structure for tert-butyl
Q32: Which of the following molecules contains a
Q33: A carbon-hydrogen bond in ethane (CH3CH3) is
Q35: The formal charge on nitrogen in the
Q38: Which of the following are correct Lewis
Q38: Within a given row of the periodic
Q40: Write a Lewis structure for a compound
Q40: The compound methylamine, CH3NH2, contains a C-N
Q41: Draw the other important resonance form of:
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents