One or more of the atoms in the structure shown should have nonzero formal charges. Redraw the structure and indicate any such charges. 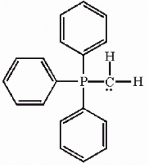
Correct Answer:
Verified
Q20: Draw the Lewis structure of acetic acid,
Q22: Assign the correct formal charge to each
Q23: Draw a proper Lewis structure for H2SO4.
Q24: Add the appropriate formal charge to each
Q26: Draw a correct Lewis structure for acetonitrile,
Q27: Add the appropriate formal charge to each
Q28: For most compounds in which a nitrogen
Q30: Draw a correct Lewis structure for boric
Q31: The electronegativity of elements on the periodic
Q36: Covalent bonds may be polar or nonpolar.
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents