It is believed that two carbon-12 nuclei can react in the core of a supergiant star to form sodium-23 and hydrogen-1.Calculate the energy released from this reaction for each mole of hydrogen formed. 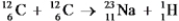 Particle Mass (amu) C-12 12.000000
Particle Mass (amu) C-12 12.000000
Na-23 22.989767
H-1 1.007825
(1 kg = 6.022 × 1026 amu;
NA = 6.022 × 1023 mol-1;
C = 2.99792458 × 108 m/s)
A) 2.16 × 1014 kJ
B) 2.16 × 1011 kJ
C) 2.16 × 108 kJ
D) 2.16 × 105 kJ
E) None of the answers is correct.
Correct Answer:
Verified
Q47: What element is the stable end-product of
Q62: What role does cadmium metal (Cd) play
Q64: Exposure to 10 nCi for 10 minutes
Q65: What is the nuclear process called where
Q66: A particle accelerator uses which of the
Q73: A 30.0-kg child receives 2.65 × 107
Q75: Sodium-21 will emit positrons, each having an
Q77: Calcium-39 undergoes positron decay. Each positron carries
Q80: Which type of radiation is the most
Q91: What is the nuclear process called in
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents