If the following electrochemical cell is constructed,what voltage will be measured on the voltmeter? 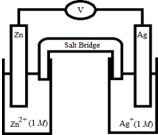 Half-Reaction E° (V) Zn2+(aq) + 2e- →Zn(s) -0.76
Half-Reaction E° (V) Zn2+(aq) + 2e- →Zn(s) -0.76
Ag+(aq) + e- → Ag(s) +0.80
A) 2.36 V
B) 1.56 V
C) 0.04 V
D) -0.04 V
E) -2.36 V
Correct Answer:
Verified
Q83: Which of the following is correct?
A) total
Q86: Which element is associated with the term
Q89: Based on the following electrochemical cell,which statement
Q90: What product forms at the cathode during
Q92: Based on the following electrochemical cell,what is
Q93: What is the standard free-energy change for
Q95: What product forms at the anode during
Q98: Aluminum does not corrode in the same
Q98: Which of the following elements can be
Q100: Which equation is correct?
A) Ecell = RT
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents