Based on the following electrochemical cell,what is the standard reduction potential of metal M at 298 K? 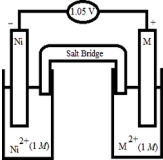 Half-Reaction E° (V) Ni2+(aq) + 2e- →Ni(s) -0.25
Half-Reaction E° (V) Ni2+(aq) + 2e- →Ni(s) -0.25
A) -1.40 V
B) -0.80 V
C) +0.25 V
D) +0.80 V
E) +1.40 V
Correct Answer:
Verified
Q83: Which of the following is correct?
A) total
Q85: A current of 250. A flows for
Q86: Which element is associated with the term
Q87: Which electrochemical cell pictured below corresponds to
Q89: Based on the following electrochemical cell,which statement
Q93: What is the standard free-energy change for
Q94: If the following electrochemical cell is constructed,what
Q95: What product forms at the anode during
Q98: Aluminum does not corrode in the same
Q98: Which of the following elements can be
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents