Based on the following electrochemical cell,what is the standard reduction potential of metal M at 298 K? (R = 8.314 J/K • mol,F = 96500 C/mol) 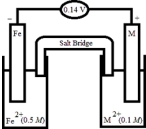 Half-Reaction E° (V) Fe2+(aq) + 2e- → Fe(s) -0.44
Half-Reaction E° (V) Fe2+(aq) + 2e- → Fe(s) -0.44
A) -0.54 V
B) +0.60 V
C) -0.30 V
D) +0.56 V
E) -0.28 V
Correct Answer:
Verified
Q72: What mass of oxygen gas is produced
Q79: What is the half-reaction that occurs at
Q81: Given Cu2+(aq)+ 2e- → Cu(s)E° = +0.34
Q83: Which is the correct cell diagram for
Q85: A current of 250. A flows for
Q87: Which electrochemical cell pictured below corresponds to
Q89: Based on the following electrochemical cell,which statement
Q91: Which component of the following cell is
Q92: Which of the following elements could be
Q95: Two cells are connected in series, so
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents