Below are energy level diagrams for two different atoms. 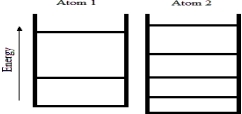 Based on the diagrams,which atom has the larger standard molar entropy at T = 298 K?
Based on the diagrams,which atom has the larger standard molar entropy at T = 298 K?
A) Atom 1 has the larger standard molar entropy because the energy of its ground state is larger than the energy of the ground state of atom 2.
B) Atom 2 has the larger standard molar entropy because the energy of its ground state is smaller than the energy of the ground state of atom 1.
C) Atom 1 has the larger standard molar entropy because it has fewer energy levels at lower energy values than atom 2.
D) Atom 2 has the larger standard molar entropy because it has more energy levels at lower energy values than atom 1.
E) Both atoms have the same value of the standard molar entropy at T = 298 K.
Correct Answer:
Verified
Q46: Is the following process spontaneous? 
Q48: Which statement is correct?
A)Oxygen is formed
Q49: Which thermodynamical property is a measure of
Q62: The absolute standard entropy of atom X(g)
Q64: For the process C6H6(l) <-----> C6H6(s) at
Q72: Which statement is correct?
A) Reaction of ADP
Q75: As the molar mass of a compound
Q77: Which of the following substances has the
Q78: At temperatures below 273 K, it is
Q100: What is the third law of thermodynamics?
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents