Below is a representation of the sparingly soluble salt MX at equilibrium in aqueous solution.(Each circle represents 1.0 ×10-3 mol of atoms,and the volume of the box is 1.0 L.Solvent water molecules are omitted for clarity.) 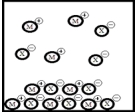 What is Ksp for MX?
What is Ksp for MX?
A) 1.5 ×10-5
B) 6.0 ×10-6
C) 3.6 ×10-1
D) 1.8 ×10-3
E) 9.0 ×10-6
Correct Answer:
Verified
Q84: Which is more soluble in an acidic
Q93: Which is more soluble in a basic
Q96: Calculate the NaOH concentration necessary to precipitate
Q98: A solution is prepared by mixing 50.0
Q99: Suppose 50.0 mL of a 0.100 M
Q103: When sodium acetate is added to a
Q107: pH = pKa + log [conjugate base]/[weak
Q109: A(n) _ is a measure of the
Q116: The _ is the point in a
Q132: _ is the way to identify the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents