Below is a titration curve for the titration of 50.0 mL of a 0.100 M solution of the weak acid HA with a 0.100 M solution of NaOH. 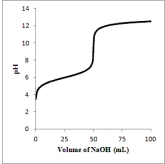 What is the approximate pKa of HA?
What is the approximate pKa of HA?
A) 4
B) 6
C) 8
D) 9
E) 12
Correct Answer:
Verified
Q84: Which is more soluble in an acidic
Q86: Suppose 50.0 mL of a 0.100 M
Q86: Which is more soluble in a basic
Q87: Which is more soluble in an acidic
Q89: A mixture made of 100 mL of
Q90: What is the name of the principle
Q91: When an acid, HA, is titrated with
Q92: The amount of strong acid added to
Q94: Which will precipitate first when AgNO3 is
Q100: After 50.0 mL of a 0.100 M
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents