For the endothermic reaction A2(g)  2A(g) ,a snapshot of an equilibrium mixture of A(g) and A2(g) at low temperature may look as follows.(Each circle represents 1.0 mol of A atoms,and the volume of the box is 1.0 L.)
2A(g) ,a snapshot of an equilibrium mixture of A(g) and A2(g) at low temperature may look as follows.(Each circle represents 1.0 mol of A atoms,and the volume of the box is 1.0 L.) 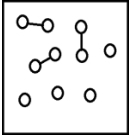 If the system pressure is lowered,what might the new equilibrium system look like?
If the system pressure is lowered,what might the new equilibrium system look like?
A) 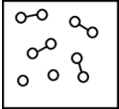
B) 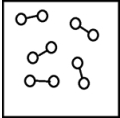
C) 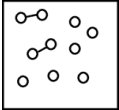
D) 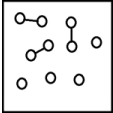
E) 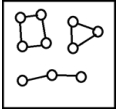
Correct Answer:
Verified
Q9: For any reaction, if ΔG° > 0,
Q13: When a reaction system reaches equilibrium, the
Q97: For the reaction 2NOCl(g) Q98: Consider this reaction at equilibrium at a Q99: In which of these gas-phase equilibria is Q100: Suppose 50.0 g of N2O4 is introduced Q101: Solid ammonium hydrogen sulfide is introduced into Q104: When the reaction 2H2S(g) Q105: For the endothermic reaction A2 (g) Q107: For the endothermic reaction A2(g) ![]()
![]()

Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents