Consider the phase diagram shown below. 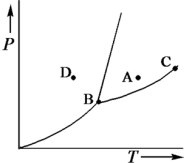 a.What phase(s)is/are present at point A?
a.What phase(s)is/are present at point A?
b.What phase(s)is/are present at point B?
c.Name point C and explain its significance.
d.Starting at D,if the pressure is lowered while the temperature remains constant,describe what will happen.
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q81: a. Name the two unit cells which
Q94: Identify the dominant (strongest)type of intermolecular force
Q99: Identify the dominant (strongest)type of intermolecular force
Q112: Octane, C8H18, boils at 125°C, whereas water
Q117: _ _ are the attractions that hold
Q125: a.State the essential requirements for hydrogen bonding
Q126: Polyethylene plastic consists of long chains of
Q128: Crystals of elemental sulfur are easily crushed,
Q137: _ is the name given to the
Q145: The shape of the water-to-glass meniscus results
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents