For different structural arrangements of atoms having the formula XeF2Cl2,which structures represent polar molecules? (Black = Xe,Yellow = F,Green = Cl) 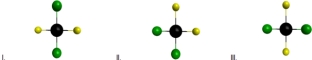
A) I and III
B) II only
C) I,II and III
D) II and III
E) None of these structures are polar.
Correct Answer:
Verified
Q18: The angles between sp2 hybrid orbitals are
Q91: According to molecular orbital (MO) theory, the
Q101: Thiocarbonyl disulfide (CSF2),based on the coordinate axes
Q102: The bond angle for an sp hybrid
Q103: Which formula is incorrectly matched with its
Q104: Which is true for carbon monoxide?
A)CO has
Q105: According to molecular orbital theory, all diatomic
Q112: Which statement is not true of molecular
Q114: In the valence bond treatment, a π
Q120: In the valence bond treatment, overlap of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents