The Lewis structure of formaldehyde,CH2O,is shown.Use VSEPR model to predict the molecular geometry and the H-C-H bond angle.Outline your reasoning. 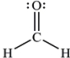
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q17: Atoms of period 3 and beyond can
Q29: In one sentence state the basic principle
Q109: Which is true concerning the molecular geometry
Q119: The bond angles in the H3O+ ion
Q121: Water has _ (number) lone pair(s) of
Q127: The number of lone pairs on the
Q128: According to the VSEPR model, a molecule
Q129: How many π bonds are there in
Q137: Pi bonds are covalent bonds in which
Q138: Use the VSEPR model to predict the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents