Suppose a 0.500-g sample of an organic compound is analyzed via bomb calorimetry.The temperature of the calorimeter is measured over time.At t = 5 min,the combustion reaction is initiated.Below is a plot of the data that are obtained. 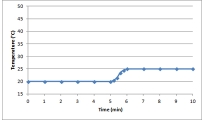 Suppose the experiment is repeated under identical conditions,but with a 1.000-g sample of the organic compound.What might a plot of the resulting data look like?
Suppose the experiment is repeated under identical conditions,but with a 1.000-g sample of the organic compound.What might a plot of the resulting data look like?
A) 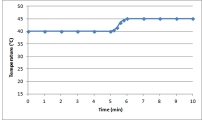
B) 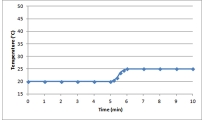
C) 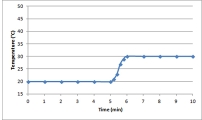
D) 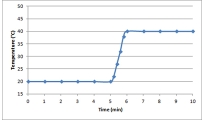
E) 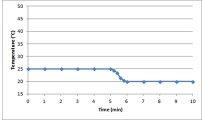
Correct Answer:
Verified
Q28: Cold packs, whose temperatures are lowered when
Q103: Chemical reactions in a bomb calorimeter occur
Q105: Which represents the formation reaction for XeF4(g)?
Q105: The enthalpy of vaporization of a compound
Q107: Consider the following two representations of chemical
Q108: All elements in their standard state have
Q108: Atoms A and Z may form either
Q108: A home aquarium is an example of
Q109: Which of the following is incorrectly matched?
A)
Q113: The work done on the surroundings by
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents