Arrange the molecules below in order of increasing solubility in water. 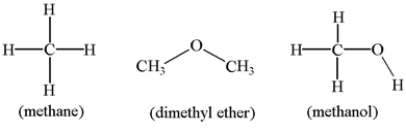
A) dimethyl ether < methane < methanol
B) dimethyl ether < methanol < methane
C) methane < methanol< dimethyl ether
D) methanol < dimethyl ether < methane
E) methane < dimethyl ether < methanol
Correct Answer:
Verified
Q31: All of the following are colligative properties
Q32: Arrange the molecules below in order of
Q33: What is the equilibrium partial pressure of
Q34: The Henry's law constant for the solubility
Q35: What concentration unit is necessary for the
Q38: If 77.5 g of ethylene glycol (HOCH2CH2OH)is
Q39: What concentration unit is necessary for the
Q40: A substance that dissolves in water and
Q41: At 25°C,what is the osmotic pressure of
Q59: What mass of ethylene glycol,when mixed with
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents