Magnesium hydroxide is used in several antacid formulations. When it is added to water it dissociates into magnesium and hydroxide ions. 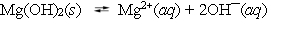 The equilibrium constant at 25 C is 8.9 *10¯12. One hundred grams of magnesium hydroxide is added to 1.00 L of water and equilibrium is established. What happens to the solution if another 10 grams of Mg(OH) 2 are now added to the mixture?
The equilibrium constant at 25 C is 8.9 *10¯12. One hundred grams of magnesium hydroxide is added to 1.00 L of water and equilibrium is established. What happens to the solution if another 10 grams of Mg(OH) 2 are now added to the mixture?
A) The hydroxide ion concentration will decrease.
B) The hydroxide ion concentration will increase.
C) The hydroxide ion concentration will be unchanged.
D) The solution will become supersaturated.
E) None of these conclusions is justified without additional information.
Correct Answer:
Verified
Q60: Compounds A, B, and C react
Q61: Magnesium carbonate dissociates to magnesium oxide
Q62: Nitrogen dioxide can dissociate to nitric oxide
Q63: The following reaction is at equilibrium at
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents