Ethane can be formed by reacting acetylene with hydrogen. 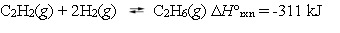 Under which reaction conditions would you expect to have the greatest equilibrium yield of ethane?
Under which reaction conditions would you expect to have the greatest equilibrium yield of ethane?
A) high temperature, high pressure
B) low temperature, high pressure
C) high temperature, low pressure
D) low temperature, low pressure
E) None of these choices is correct, unless a catalyst is present.
Correct Answer:
Verified
Q64: The reaction system Q65: Magnesium hydroxide is used in several Q66: The reaction of nitric oxide to Q67: At 450 Q68: Sodium hydrogen carbonate decomposes above 110 Q70: Hydrogen bromide will dissociate into hydrogen Q71: Stearic acid, nature's most common fatty Q72: The reaction system Q73: Methanol can be synthesized by combining carbon Q74: Hydrogen sulfide can be formed in the![]()
![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents