The reaction, 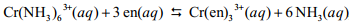 , where en represents ethylenediamine, has a small value for the enthalpy change, Hrxn, yet the free-energy change is large because
, where en represents ethylenediamine, has a small value for the enthalpy change, Hrxn, yet the free-energy change is large because
A) the reaction rate is fast.
B) the entropy change is large and positive.
C) the enthalpy change is large enough to matter.
D) the entropy change is large and negative.
E) ethylenediamine has amino groups that are stronger bases than ammonia.
Correct Answer:
Verified
Q54: When the five 3d orbitals on a
Q55: Which d orbitals have a higher energy
Q56: Which statement describing the splitting of metal
Q57: The interaction of a metal ion with
Q57: Crystal field theory describes _
A)how ligands cause
Q58: Nickel ions, Ni2+, should have the greatest
Q62: Co(NH3)63+(aq) absorbs light in the blue region
Q63: How many unpaired electrons are there in
Q64: How many d electrons are there in
Q64: Which of the following complexes has the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents