Which d orbitals have a higher energy in an octahedral complex?
A) dxy and dxz
B) dz2 and dyz
C) dz2 and 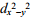
D) 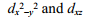
E) dxy, dxz, and dyz
Correct Answer:
Verified
Q25: EDTA is an example of a _
Q40: How many chelation sites and donor groups
Q51: Oxalic acid is a dicarboxylic acid with
Q53: Which d orbitals) is/are highest in energy
Q54: When the five 3d orbitals on a
Q56: Which statement describing the splitting of metal
Q57: The interaction of a metal ion with
Q57: Crystal field theory describes _
A)how ligands cause
Q58: Nickel ions, Ni2+, should have the greatest
Q59: The reaction, ![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents