Which d orbitals) is/are highest in energy in a tetrahedral complex?
A) dz2
B) dxy, dxz, and dyz
C) dz2 and 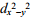
D) 11ee34ea_62da_ec1f_b797_4f3e5b0d5d55_TB6562_00
E) dxy and dxz
Correct Answer:
Verified
Q25: EDTA is an example of a _
Q40: How many chelation sites and donor groups
Q48: The dxy, dxz, and dyz orbitals are
Q49: Which of the following is a chelation
Q51: Oxalic acid is a dicarboxylic acid with
Q54: When the five 3d orbitals on a
Q55: Which d orbitals have a higher energy
Q56: Which statement describing the splitting of metal
Q57: The interaction of a metal ion with
Q58: Nickel ions, Ni2+, should have the greatest
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents