Which of the following solids will provide the greatest concentration of Cl1 − ions if one mole of the salt is placed in one liter of deionized water?
A) AgCl( s ) 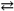 Ag1+( aq ) + Cl1 − ( aq ) Ksp = 1.8 × 10 − 10
Ag1+( aq ) + Cl1 − ( aq ) Ksp = 1.8 × 10 − 10
B) PbCl2( s ) 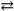 Pb2+( aq ) + 2 Cl1 − ( aq ) Ksp = 1.75 × 10 − 5
Pb2+( aq ) + 2 Cl1 − ( aq ) Ksp = 1.75 × 10 − 5
C) Hg2Cl2( s ) 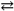 2 Hg1+( aq ) + 2 Cl1 − ( aq ) Ksp = 1.1 × 10 − 18
2 Hg1+( aq ) + 2 Cl1 − ( aq ) Ksp = 1.1 × 10 − 18
D) NaCl( s ) → Na1+( aq ) + Cl1 − ( aq )
Correct Answer:
Verified
Q29: What is the pH of a 0.200
Q30: The synthesis of the pesticide thionyl chloride
Q31: Which of the following is a weak
Q32: Consider the solubility of BaSO4. If Ba(NO3)2
Q33: Potassium hydrogen phthalate (KHP) concentrations can be
Q35: What is the pH of a 0.1055
Q36: Which of following species is amphoteric?
A) NaOH
B)
Q37: The lower the pH of a weak
Q38: The conjugate base of ammonium chloride is:
A)
Q39: At equilibrium Δ G is:
A) = 0
B)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents