What change in the reaction direction occurs if dilute NaOH is added to a H2PO41- solution?
H2PO4 1− + H2O 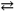 HPO42 − + H3O+
HPO42 − + H3O+
A) shifts to the right
B) shifts to the left
C) no change in the reaction
D) there is insufficient information to solve this problem
Correct Answer:
Verified
Q23: What change in reaction direction occurs if
Q24: What is the pH of a 0.150
Q25: What is the pH of a 0.0250
Q26: What is the molar solubility of CaF2
Q27: Which of the following is a strong
Q29: What is the pH of a 0.200
Q30: The synthesis of the pesticide thionyl chloride
Q31: Which of the following is a weak
Q32: Consider the solubility of BaSO4. If Ba(NO3)2
Q33: Potassium hydrogen phthalate (KHP) concentrations can be
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents