When natural gas (predominantly methane, CH4) burns in air. The following reaction occurs. How much energy is involved in the combustion of 13.0 g of methane? 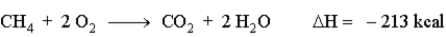
A) 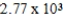 kcal
kcal
B) 16.4 kcal
C) 173 kcal
D) 0.979 kcal
Correct Answer:
Verified
Q22: Which of the following most closely defines
Q24: The Haber Process is a method for
Q26: Consider the following reaction.
NaOH(aq) + H2SO4(aq)
Q31: If the reaction given below occurs and
Q31: In the equation for an exothermic reaction,
Q32: A block of iron forming a pool
Q35: How many moles of N2 are required
Q36: Which of the following statements is not
Q37: Consider the following generic reaction.
2 A(s)
Q40: The reactants in a chemical reaction are
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents