If the reaction given below occurs and pure A and B were mixed, which of the following would take place as equilibrium was established? A + B 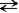 C
C
A) The concentration of C would increase for a time, then remain constant.
B) The concentration of A would increase for a time, then decrease.
C) The concentration of B would increase for a time, then remain constant.
D) Both a and c will occur.
Correct Answer:
Verified
Q22: Which of the following most closely defines
Q24: The Haber Process is a method for
Q26: Consider the following reaction: Q26: Consider the following reaction. Q31: In the equation for an exothermic reaction, Q32: A block of iron forming a pool Q32: When natural gas (predominantly methane, CH4) burns Q35: How many moles of N2 are required Q36: Which of the following statements is not Q40: The reactants in a chemical reaction are![]()
NaOH(aq) + H2SO4(aq)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents