Passage
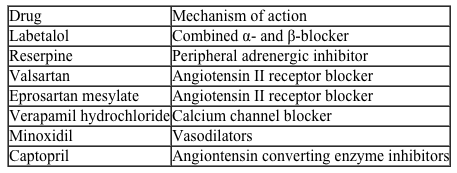
High blood pressure, or hypertension, is a prevalent condition in the United States. One in three adults has hypertension, and approximately 20% do not know that they have it. If left untreated, high blood pressure can lead to major health issues, including stroke, heart attack, and kidney disease or failure. A number of drugs can help control hypertension, including those shown in Table 1. These drugs can be classified into different groups based on their mechanism of action.Table 1 Drugs Used to Treat High Blood Pressure
 Labetalol (Figure 1) exists as four stereoisomers and is administered as a mixture of all four. This drug can be classified as a pseudohybrid drug because two of the isomers, (R,R) and (S,R) , exhibit biological activity; the other two isomers, (S,S) and (R,S) , are not active. The (R,R) isomer acts as a nonselective β-adrenergic receptor blocker, and the (S,R) isomer acts as a selective α-adrenergic receptor blocker.
Labetalol (Figure 1) exists as four stereoisomers and is administered as a mixture of all four. This drug can be classified as a pseudohybrid drug because two of the isomers, (R,R) and (S,R) , exhibit biological activity; the other two isomers, (S,S) and (R,S) , are not active. The (R,R) isomer acts as a nonselective β-adrenergic receptor blocker, and the (S,R) isomer acts as a selective α-adrenergic receptor blocker.
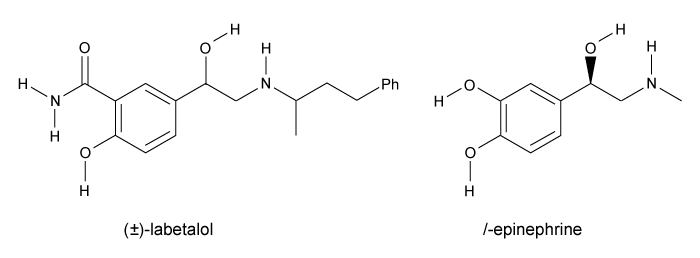 Figure 1 Structure of (±) -labetalol and l-epinephrinel-epinephrine (Figure 1) is the agonist of the α- and β-adrenergic receptors. The structural motifs that allow l-epinephrine to bind to the adrenergic receptors include an amine separated from an aromatic ring by two carbon units, a hydroxyl group at a chiral center beta to the amine, and two hydroxyl groups on the aromatic ring in the meta and para positions. With a few structural modifications, the agonist l-epinephrine can be converted into the antagonist labetalol. Substitution of a hydroxyl on the aromatic ring with an amide group and extension of the methyl group on the amine transforms l-epinephrine into labetalol. These substitutions keep a majority of the structural motifs necessary for labetalol to mimic l-epinephrine and bind to the adrenergic receptors.
Figure 1 Structure of (±) -labetalol and l-epinephrinel-epinephrine (Figure 1) is the agonist of the α- and β-adrenergic receptors. The structural motifs that allow l-epinephrine to bind to the adrenergic receptors include an amine separated from an aromatic ring by two carbon units, a hydroxyl group at a chiral center beta to the amine, and two hydroxyl groups on the aromatic ring in the meta and para positions. With a few structural modifications, the agonist l-epinephrine can be converted into the antagonist labetalol. Substitution of a hydroxyl on the aromatic ring with an amide group and extension of the methyl group on the amine transforms l-epinephrine into labetalol. These substitutions keep a majority of the structural motifs necessary for labetalol to mimic l-epinephrine and bind to the adrenergic receptors.
-(S,R) - and (R,R) -labetalol, the active forms of the drug, can be described as which of the following?DiastereomersEnantiomersConformational isomers
A) I only
B) I and II only
C) II and III only
D) I, II, and III
Correct Answer:
Verified
Q25: Passage
The cryptophycins, isolated from cyanobacteria, are a
Q26: Passage
Aldehydes are organic compounds encountered in everyday
Q27: Passage
Aldehydes are organic compounds encountered in everyday
Q28: Passage
Aldehydes are organic compounds encountered in everyday
Q29: Passage
The cryptophycins, isolated from cyanobacteria, are a
Q31: Passage
Aldehydes are organic compounds encountered in everyday
Q32: Passage
Two key ingredients found in many soaps
Q33: Passage
Two key ingredients found in many soaps
Q34: Passage
The cryptophycins, isolated from cyanobacteria, are a
Q35: Passage
Aldehydes are organic compounds encountered in everyday
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents