Passage
Aldehydes are organic compounds encountered in everyday life and used as intermediates in metabolism. Common aldehydes include formaldehyde, which is used in the fixation of tissues, and cinnamaldehyde, which provides the flavor and smell of cinnamon (Figure 1) .
 Figure 1 Common aldehydesThe aldol condensation shown in Figure 2 is an important carbon-carbon bond-forming reaction that has many applications in the synthesis of bioactive molecules. It also takes place in some metabolic processes, such as the citric acid cycle and gluconeogenesis. The aldol condensation begins with the aldol reaction, which can be acid- or base-catalyzed and requires nucleophilic addition to a carbonyl to provide the aldol product, which can be a β-hydroxy ketone or aldehyde. The final step of the aldol condensation consists of an acid- or base-catalyzed dehydration of the aldol product to yield an α,β-unsaturated ketone or aldehyde.
Figure 1 Common aldehydesThe aldol condensation shown in Figure 2 is an important carbon-carbon bond-forming reaction that has many applications in the synthesis of bioactive molecules. It also takes place in some metabolic processes, such as the citric acid cycle and gluconeogenesis. The aldol condensation begins with the aldol reaction, which can be acid- or base-catalyzed and requires nucleophilic addition to a carbonyl to provide the aldol product, which can be a β-hydroxy ketone or aldehyde. The final step of the aldol condensation consists of an acid- or base-catalyzed dehydration of the aldol product to yield an α,β-unsaturated ketone or aldehyde.
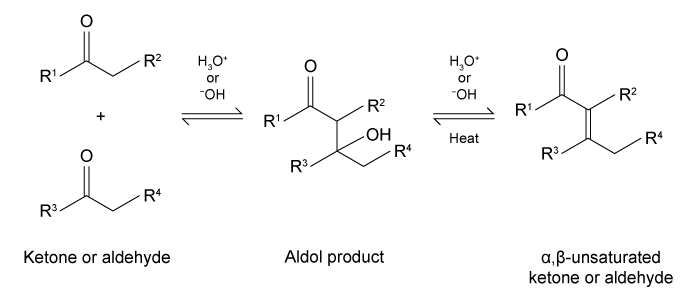 Figure 2 General aldol reaction and condensationThe reverse of an aldol reaction or condensation is known as a retro-aldol, which is important in metabolism, most notably in glycolysis. This reaction breaks a carbon-carbon bond to form aldehydes and/or ketones. In glycolysis, fructose 1,6-bisphosphate is broken down into an aldehyde and a ketone, glyceraldehyde 3-phosphate (GAP) and dihydroxyacetone phosphate (DHAP) , respectively (Figure 3) .
Figure 2 General aldol reaction and condensationThe reverse of an aldol reaction or condensation is known as a retro-aldol, which is important in metabolism, most notably in glycolysis. This reaction breaks a carbon-carbon bond to form aldehydes and/or ketones. In glycolysis, fructose 1,6-bisphosphate is broken down into an aldehyde and a ketone, glyceraldehyde 3-phosphate (GAP) and dihydroxyacetone phosphate (DHAP) , respectively (Figure 3) .
 Figure 3 Retro-aldol in glycolysis
Figure 3 Retro-aldol in glycolysis
-Which of the following reagents CANNOT be used to make benzoic acid from benzaldehyde?
A) CrO3
B) KMnO4
C) H2CrO4
D) PCC
Correct Answer:
Verified
Q21: Passage
High blood pressure, or hypertension, is a
Q22: Passage
The cryptophycins, isolated from cyanobacteria, are a
Q23: Passage
Two key ingredients found in many soaps
Q24: Passage
High blood pressure, or hypertension, is a
Q25: Passage
The cryptophycins, isolated from cyanobacteria, are a
Q27: Passage
Aldehydes are organic compounds encountered in everyday
Q28: Passage
Aldehydes are organic compounds encountered in everyday
Q29: Passage
The cryptophycins, isolated from cyanobacteria, are a
Q30: Passage
High blood pressure, or hypertension, is a
Q31: Passage
Aldehydes are organic compounds encountered in everyday
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents