Passage
Two key ingredients found in many soaps are PEG 150 distearate and ethylhexyl glycerin. Both can be synthesized from glyceryl tristearate (Figure 1) .
 Figure 1 Formation of PEG 150 distearate and ethylhexyl glycerin from glyceryl tristearateThe first step in this process requires the saponification of glyceryl tristearate. The resulting stearate molecules can then react with PEG 150 in an acid-catalyzed Fischer esterification to form PEG 150 distearate. The glycerol produced during saponification is used as a nucleophile to form ethylhexyl glycerin through a base-catalyzed SN2 reaction with an ethylhexyl halide.Studies have shown that hand washing with soap can substantially reduce the number of germs present on hands and that washing hands before preparing food is particularly effective in reducing illness. Researchers examined the effect of washing for 20 seconds with different amounts of soap containing PEG 150 distearate and ethylhexyl glycerin and found an inverse correlation between the volume of soap used and the amount of bacteria recovered from the hands. The data are shown in Figure 2.
Figure 1 Formation of PEG 150 distearate and ethylhexyl glycerin from glyceryl tristearateThe first step in this process requires the saponification of glyceryl tristearate. The resulting stearate molecules can then react with PEG 150 in an acid-catalyzed Fischer esterification to form PEG 150 distearate. The glycerol produced during saponification is used as a nucleophile to form ethylhexyl glycerin through a base-catalyzed SN2 reaction with an ethylhexyl halide.Studies have shown that hand washing with soap can substantially reduce the number of germs present on hands and that washing hands before preparing food is particularly effective in reducing illness. Researchers examined the effect of washing for 20 seconds with different amounts of soap containing PEG 150 distearate and ethylhexyl glycerin and found an inverse correlation between the volume of soap used and the amount of bacteria recovered from the hands. The data are shown in Figure 2.
 Figure 2 Bacteria recovered from hands after washing with varying amounts of soapThe researchers then investigated the effect of hand washing with different kinds of soap on the transfer of bacteria from hands to objects. Volunteers washed with a fixed volume of soap for 30 seconds, then handled sterilized plastic spheres for 30 seconds. Bacteria were then recovered from the spheres. The results are shown in Table 1.Table 1 Bacteria Recovered from Objects
Figure 2 Bacteria recovered from hands after washing with varying amounts of soapThe researchers then investigated the effect of hand washing with different kinds of soap on the transfer of bacteria from hands to objects. Volunteers washed with a fixed volume of soap for 30 seconds, then handled sterilized plastic spheres for 30 seconds. Bacteria were then recovered from the spheres. The results are shown in Table 1.Table 1 Bacteria Recovered from Objects
 Adapted from Fuls JL, Rodgers ND, Fischler GE, et al. Alternative hand contamination technique to compare the activities of antimicrobial and nonantimicrobial soaps under different test conditions. Appl Environ Microbiol. 2008.
Adapted from Fuls JL, Rodgers ND, Fischler GE, et al. Alternative hand contamination technique to compare the activities of antimicrobial and nonantimicrobial soaps under different test conditions. Appl Environ Microbiol. 2008.
-Researchers produced ethylhexyl glycerin using a partially deuterated ethylhexyl halide and generated the product shown below. 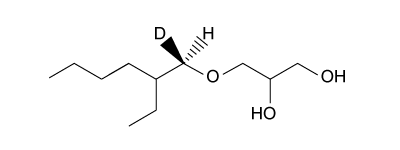 What was the configuration of the deuterated carbon prior to the reaction?
What was the configuration of the deuterated carbon prior to the reaction?
A) S, because the configuration is inverted by the reaction
B) R, because the configuration is inverted by the reaction
C) S, because the configuration is preserved by the reaction
D) R, because the configuration is preserved by the reaction
Correct Answer:
Verified
Q18: Passage
High blood pressure, or hypertension, is a
Q19: Passage
The drug paracetamol, also known as acetaminophen,
Q20: Passage
A limited number of cellular functions exist
Q21: Passage
High blood pressure, or hypertension, is a
Q22: Passage
The cryptophycins, isolated from cyanobacteria, are a
Q24: Passage
High blood pressure, or hypertension, is a
Q25: Passage
The cryptophycins, isolated from cyanobacteria, are a
Q26: Passage
Aldehydes are organic compounds encountered in everyday
Q27: Passage
Aldehydes are organic compounds encountered in everyday
Q28: Passage
Aldehydes are organic compounds encountered in everyday
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents