Passage
The cryptophycins, isolated from cyanobacteria, are a family of macrocyclic molecules. Researchers have studied the biological activity of these compounds and found that many of them are active against multi-drug-resistant tumor cells.Cryptophycin-52, an analogue of the natural cryptophycins, has been tested in clinical trials for its efficacy against cancer cells, but the studies were suspended due to neurotoxic side effects. Nevertheless, the synthesis of similar analogues continues to be of interest in cancer research. A key step in the synthesis of cryptophycin-52 is the stereospecific formation of an epoxide from an alkene and oxone (Figure 1) , which can be facilitated by the Shi catalyst. The Shi catalyst (Figure 2) is derived from the carbohydrate D-fructose and is known among organic chemists for its ability to form epoxides in hydrocarbons in a stereospecific manner.
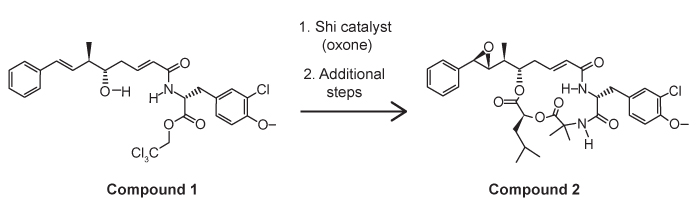 Figure 1 Shi epoxidation of compound 1
Figure 1 Shi epoxidation of compound 1
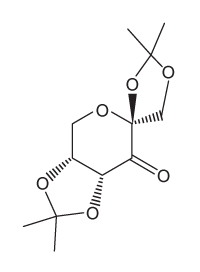 Figure 2 Structure of the Shi catalystThe epoxidation of compound 1 was monitored by thin-layer chromatography (TLC) . When the reaction was completed, high-performance liquid chromatography (HPLC) was used to analyze the epoxide product. A chiral column that had an affinity for the desired epoxide was used. A standard of the desired epoxide was obtained to optimize the conditions and determine the retention time of the epoxide. The HPLC chromatogram of the epoxide standard is shown in Figure 3.
Figure 2 Structure of the Shi catalystThe epoxidation of compound 1 was monitored by thin-layer chromatography (TLC) . When the reaction was completed, high-performance liquid chromatography (HPLC) was used to analyze the epoxide product. A chiral column that had an affinity for the desired epoxide was used. A standard of the desired epoxide was obtained to optimize the conditions and determine the retention time of the epoxide. The HPLC chromatogram of the epoxide standard is shown in Figure 3.
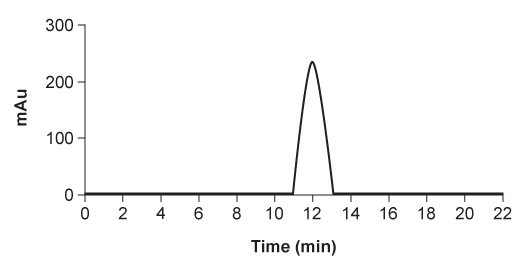 Figure 3 HPLC of epoxide standard
Figure 3 HPLC of epoxide standard
Adapted from Weiß C, Bogner T, Sammet B, Sewald N. Total synthesis and biological evaluation of fluorinated cryptophycins. Beilstein J Org Chem. 2012.
-After isolation of compound 1, an infrared spectrum was obtained. The spectrum gives all of the following information EXCEPT:
A) the spin-spin splitting of atoms in a compound.
B) the signals corresponding to stretching vibrations and rotations.
C) the amount of light absorbed at a certain frequency.
D) the relative amount of energy needed to stretch a bond.
Correct Answer:
Verified
Q17: Passage
Hemophilia B is a blood clotting disorder
Q18: Passage
High blood pressure, or hypertension, is a
Q19: Passage
The drug paracetamol, also known as acetaminophen,
Q20: Passage
A limited number of cellular functions exist
Q21: Passage
High blood pressure, or hypertension, is a
Q23: Passage
Two key ingredients found in many soaps
Q24: Passage
High blood pressure, or hypertension, is a
Q25: Passage
The cryptophycins, isolated from cyanobacteria, are a
Q26: Passage
Aldehydes are organic compounds encountered in everyday
Q27: Passage
Aldehydes are organic compounds encountered in everyday
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents