Passage
Ethanol is an important source of energy that is frequently used as a supplement to gasoline. It can be obtained from renewable resources such as the cellulose found in plant matter. When subjected to a process called gasification, the plant mass is converted to a mixture called syngas, composed predominantly of carbon monoxide and molecular hydrogen. Syngas can be passed over a rhodium catalyst to generate ethanol and other products. After using this method, a group of researchers analyzed the resulting products by gas chromatography using helium as a carrier gas. The results are shown in Figure 1.
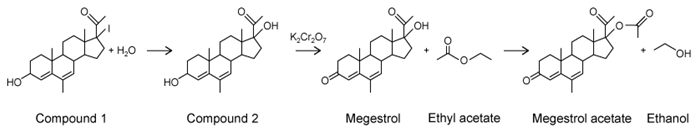
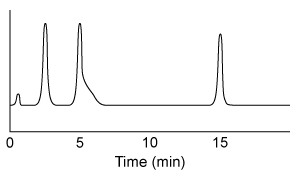 Figure 1 Gas chromatograph of syngas catalysis productsMass spectrometry revealed that the process yielded similar amounts of ethanol, acetic acid, and acetaldehyde, with trace amounts of ethane. In addition, the ethanol peak was found to be contaminated with ethyl acetate. Another group of researchers in the same laboratory had recently produced Compound 1 as a by-product in a separate reaction. They combined Compound 1 with purified ethyl acetate to form megestrol acetate, a drug used in the treatment of certain cancers. This reaction also produced ethanol, as shown in Scheme 1.
Figure 1 Gas chromatograph of syngas catalysis productsMass spectrometry revealed that the process yielded similar amounts of ethanol, acetic acid, and acetaldehyde, with trace amounts of ethane. In addition, the ethanol peak was found to be contaminated with ethyl acetate. Another group of researchers in the same laboratory had recently produced Compound 1 as a by-product in a separate reaction. They combined Compound 1 with purified ethyl acetate to form megestrol acetate, a drug used in the treatment of certain cancers. This reaction also produced ethanol, as shown in Scheme 1.
 Scheme 1
Scheme 1
Adapted from Lopez L, Velasco J, Montes V, Marinas A, Cabrera S, Boutonnet M, Järås S. Catalysts 2015.
-During the conversion of Compound 2 to megestrol, why was the secondary alcohol affected, whereas the tertiary alcohol was not?
A) Tertiary alcohols cannot be readily oxidized.
B) The secondary alcohol is stabilized by resonance.
C) The tertiary alcohol is less acidic than the secondary alcohol.
D) Secondary alcohols are better nucleophiles.
Correct Answer:
Verified
Q51: Passage
Two key ingredients found in many soaps
Q52: Passage
Ethanol is an important source of energy
Q53: Passage
Two key ingredients found in many soaps
Q54: Passage
Ethanol is an important source of energy
Q55: Passage
Ethanol is an important source of energy
Q57: Passage
Two key ingredients found in many soaps
Q58: Which structure is the product of the
Q59: Passage
The β-lactam scaffold is an important feature
Q60: Passage
Two key ingredients found in many soaps
Q61: If an alcohol were to undergo a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents