Passage
The β-lactam scaffold is an important feature in a class of broad-spectrum antibiotics that includes the penicillins, cephalosporins, and monobactams. These antibiotics are used to treat a variety of diseases caused by bacteria. The penicillins work by inhibiting a step in the synthesis of peptidoglycan. However, the development of antibiotic resistance in the form of enzymes such as β-lactamase is an ongoing problem.An aromatic ring linked to the β-lactam ring has been reported to participate in hydrophobic interactions with β-lactamase active sites, and an aromatic ring bonded to the nitrogen of the β-lactam has shown to be beneficial to biological activity. With this in mind, researchers synthesized a group of penicillanic acid analogues with substituted aromatic rings bonded to the β-lactam ring in an effort to overcome the challenge of antibiotic resistance. The substituents on the aromatic rings were varied, including -OCH3 and -NO2, to make several analogues. A key step in the synthesis of these analogues was the coupling of Compounds 1 and 2 to form a compound with two β-lactam rings (Reaction 1) .
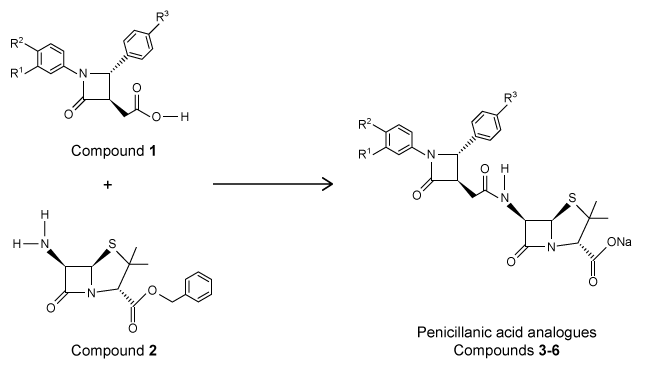 Reaction 1The researchers studied the analogues' structure-activity relationship and antimicrobial activity against the gram-negative bacteria Escherichia coli and the gram-positive bacteria Staphylococcus aureus. Ampicillin, a penicillin derivative used to treat illnesses brought about by gram-positive and gram-negative bacteria, was used as a control. The minimal inhibitory concentrations are shown in Table 1.Table 1 Minimal Inhibitory Concentration (MIC)
Reaction 1The researchers studied the analogues' structure-activity relationship and antimicrobial activity against the gram-negative bacteria Escherichia coli and the gram-positive bacteria Staphylococcus aureus. Ampicillin, a penicillin derivative used to treat illnesses brought about by gram-positive and gram-negative bacteria, was used as a control. The minimal inhibitory concentrations are shown in Table 1.Table 1 Minimal Inhibitory Concentration (MIC)
 The penicillanic acid analogues were tested for their effect on mammalian cell viability because toxicity to cells is also an important factor to consider for potential drug candidates. The general acceptable level of cell viability for a drug candidate is 70%. These results are shown in Figure 1.
The penicillanic acid analogues were tested for their effect on mammalian cell viability because toxicity to cells is also an important factor to consider for potential drug candidates. The general acceptable level of cell viability for a drug candidate is 70%. These results are shown in Figure 1.
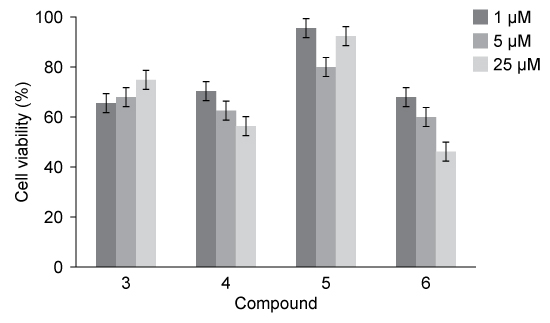 Figure 1 Cell viability assay results
Figure 1 Cell viability assay results
Adapted from De rosa M, Vigliotta G, Palma G, Saturnino C, Soriente A. Novel Penicillin-Type Analogues Bearing a Variable Substituted 2-Azetidinone Ring at Position 6: Synthesis and Biological Evaluation. Molecules. 2015.
-Which conclusion about the bioactivity of Compounds 3-6 is best supported by the data in Table 1 and Figure 1?
A) The addition of substituted aromatic rings to the penicillin core is detrimental to cell viability.
B) Compound 5 has the greatest effect on cell viability.
C) Compound 3 demonstrates the greatest antimicrobial activity against S. aureus.
D) Compared to ampicillin, Compounds 3-6 display an increased antimicrobial activity against gram-negative bacteria.
Correct Answer:
Verified
Q69: Which structure is a tautomer of guanine?
A)
Q70: Passage
Fluorescent amino acids are useful for labeling
Q71: Passage
Fluorescent amino acids are useful for labeling
Q72: Ubiquinone (shown below) is an important molecule
Q73: The molecule (3R,4S)-3-Bromo-4-chlorocyclohexanone is shown below:
Q75: Researchers want to synthesize valine from isovaleric
Q76: Which of the following amino acids would
Q77: Passage
Fluorescent amino acids are useful for labeling
Q78: The (R)-enantiomer of the antiasthma drug albuterol
Q79: A solution of Compound 1, shown below,
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents