A solution of Compound 1, shown below, absorbs light maximally at 448 nm in the absence of copper(II) ions but shifts to a 623 nm absorption maximum upon the addition of Cu2+. Which of the following best describes this process? 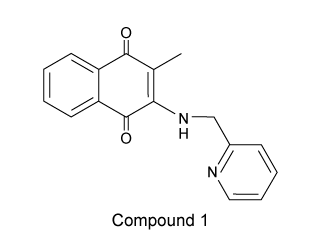
A) Changes in electronic structure cause the solution to change from yellow to blue.
B) Changes in vibrational modes cause the solution to change from green to yellow.
C) Changes in the mass-to-charge ratio (m/z) cause the solution to change from violet to orange.
D) Changes in nuclear spin cause the solution to change from colorless to violet.
Correct Answer:
Verified
Q74: Passage
The β-lactam scaffold is an important feature
Q75: Researchers want to synthesize valine from isovaleric
Q76: Which of the following amino acids would
Q77: Passage
Fluorescent amino acids are useful for labeling
Q78: The (R)-enantiomer of the antiasthma drug albuterol
Q80: Passage
Fluorescent amino acids are useful for labeling
Q81: Passage
Electrospray ionization mass spectrometry (ESI-MS) employs a
Q82: Passage
Electrospray ionization mass spectrometry (ESI-MS) employs a
Q83: Passage
A group of students studied the reactivity
Q84: Passage
A group of students studied the reactivity
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents