Passage
A group of students studied the reactivity of four alkyl halides (R−X) in two nucleophilic substitution reactions. Nucleophilic substitution reactions can go through one of two mechanisms. The SN2 reaction requires substitution of a leaving group with a nucleophile in a single step (Reaction 1) , and the SN1 reaction requires elimination of the leaving group and formation of an intermediate before nucleophilic substitution can occur, resulting in a two-step process (Reaction 2) .R−X + NaI → R−I + NaX↓Reaction 1R−X + AgNO3 → R+ + AgX↓ + HOCH2CH3 → R−OCH2CH3 + HNO3Reaction 2Experiment 1The students performed the SN2 reaction (Reaction 1) by the addition of a few drops of each alkyl halide into a separate test tube containing 1 mL of 15% solution of NaI in acetone at 25°C. After mixing, they monitored the reaction for the formation of a precipitate (sodium halide salt) . If no precipitate formed after 5 minutes, they raised the temperature of the reaction to 50°C and again checked for the formation of a precipitate. Formation of a precipitate indicated the substrate underwent nucleophilic substitution. The results observed for the Finkelstein reaction are shown in Table 1.Table 1 Results of the SN2 Reaction
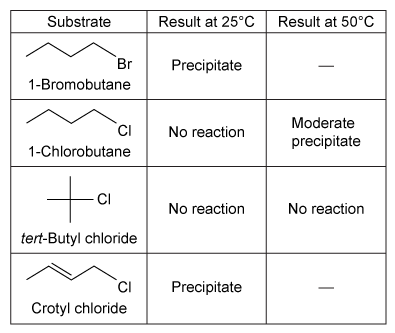 Experiment 2The students repeated the procedure followed in Experiment 1 except they added a few drops of each alkyl halide into a separate test tube containing 1 mL of 1% solution of AgNO3 in ethanol at 25°C. The results observed for this reaction are shown in Table 2.Table 2 Results of the SN1 Reaction
Experiment 2The students repeated the procedure followed in Experiment 1 except they added a few drops of each alkyl halide into a separate test tube containing 1 mL of 1% solution of AgNO3 in ethanol at 25°C. The results observed for this reaction are shown in Table 2.Table 2 Results of the SN1 Reaction
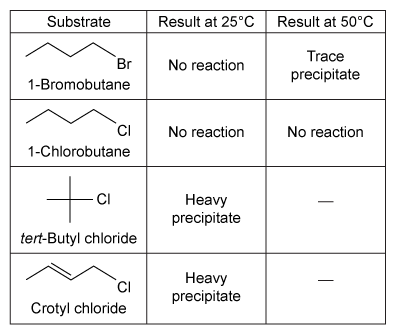
-The substrate tert-butylchloride reacts in Experiment 2 but not in Experiment 1 because:the leaving group is bonded to a primary carbon.a stable carbocation forms in Experiment 2.the leaving group is bonded to a tertiary carbon.an unstable carbocation forms in Experiment 2.
A) I and II only
B) I and IV only
C) II and III only
D) III and IV only
Correct Answer:
Verified
Q79: A solution of Compound 1, shown below,
Q80: Passage
Fluorescent amino acids are useful for labeling
Q81: Passage
Electrospray ionization mass spectrometry (ESI-MS) employs a
Q82: Passage
Electrospray ionization mass spectrometry (ESI-MS) employs a
Q83: Passage
A group of students studied the reactivity
Q85: Which of the following statements accurately describes
Q86: Passage
A group of students studied the reactivity
Q87: Passage
A group of students studied the reactivity
Q88: What structure is equivalent to Compound 1
Q89: Passage
A group of students studied the reactivity
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents