Passage Electrospray Ionization Mass Spectrometry (ESI-MS) Employs a Soft Ionization Technique
Passage
Electrospray ionization mass spectrometry (ESI-MS) employs a soft ionization technique that, unlike traditional ionization via electron bombardment, results in little to no fragmentation of target molecules. During electron bombardment, electrons collide with the analyte and cause the molecule to lose an electron whereas during ESI-MS, ions are produced by passing the analyte solution through an electrospray needle that has a potential difference applied with respect to a counterelectrode. This leads to the formation of charged droplets, which are repelled from the needle toward the counterelectrode and mass spectrometer, as shown in Figure 1.
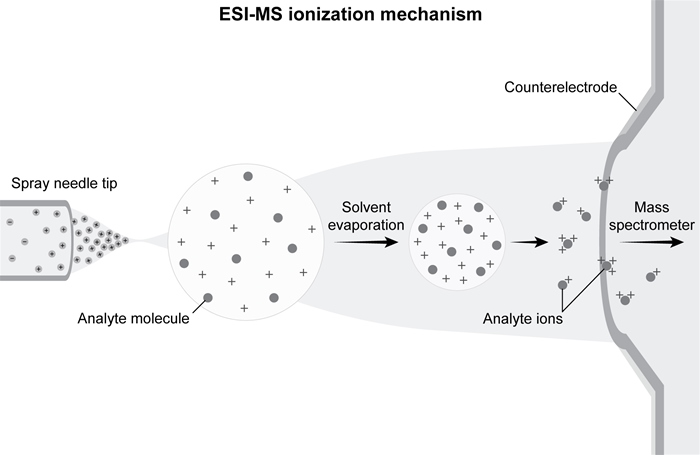 Figure 1 ESI-MS ionization mechanismDuring the droplet's flight, the solvent is evaporated off analyte molecules. Ionization occurs through the protonation of basic sites on target molecules. Reaction 1 shows the number of protons n added to molecule M.M → (M + nH) n+Reaction 1Researchers used ESI-MS to quantify the formation of four pyridyloxobutyl-DNA (POB-DNA) adducts, shown in Figure 2. POB-DNA adduct formation involves highly mutagenic pyridyloxobutylating agents, which are formed from cytochrome P450-mediated hydroxylation of 4-(methylnitrosamino) -1-(3-pyridyl) -1-butanone (NNK) , an abundant N-nitrosamine carcinogen found in cigarette smoke. Adducts were produced in vitro by treating samples of calf thymus DNA with NNK. The DNA adducts were then separated by reversed-phase high-performance liquid chromatography (RP-HPLC) and analyzed via ESI-MS.
Figure 1 ESI-MS ionization mechanismDuring the droplet's flight, the solvent is evaporated off analyte molecules. Ionization occurs through the protonation of basic sites on target molecules. Reaction 1 shows the number of protons n added to molecule M.M → (M + nH) n+Reaction 1Researchers used ESI-MS to quantify the formation of four pyridyloxobutyl-DNA (POB-DNA) adducts, shown in Figure 2. POB-DNA adduct formation involves highly mutagenic pyridyloxobutylating agents, which are formed from cytochrome P450-mediated hydroxylation of 4-(methylnitrosamino) -1-(3-pyridyl) -1-butanone (NNK) , an abundant N-nitrosamine carcinogen found in cigarette smoke. Adducts were produced in vitro by treating samples of calf thymus DNA with NNK. The DNA adducts were then separated by reversed-phase high-performance liquid chromatography (RP-HPLC) and analyzed via ESI-MS.
 Figure 2 Molecular structure of POB-DNA adducts
Figure 2 Molecular structure of POB-DNA adducts
Adapted from Lao Y, Villalta PW, Sturla SJ, Wang M, Hecht SS. Quantitation of pyridyloxobutyl DNA adducts of tobacco-specific nitrosamines in rat tissue DNA by high-performance liquid chromatography-electrospray ionization-tandem mass spectrometry. Chem Res Toxicol. 2006.
-Prior to adduct formation, DNA was isolated from a cellular mixture. Membrane lipids were first extracted using an organic solvent, which was then evaporated, and DNA was precipitated from the aqueous layer using cold ethanol and sodium acetate. Which of the following mechanisms allowed for DNA precipitation?
A) Sodium acetate lowers the pH of the solution, changing the conformation of the DNA structure.
B) Ethanol has a greater polarity than water, pulling DNA out of the aqueous solution.
C) Cold ethanol lowers the temperature of the solution, precipitating frozen DNA.
D) Sodium acetate forms ionic bonds with DNA, neutralizing its charge.
Correct Answer:
Verified
Q77: Passage
Fluorescent amino acids are useful for labeling
Q78: The (R)-enantiomer of the antiasthma drug albuterol
Q79: A solution of Compound 1, shown below,
Q80: Passage
Fluorescent amino acids are useful for labeling
Q81: Passage
Electrospray ionization mass spectrometry (ESI-MS) employs a
Q83: Passage
A group of students studied the reactivity
Q84: Passage
A group of students studied the reactivity
Q85: Which of the following statements accurately describes
Q86: Passage
A group of students studied the reactivity
Q87: Passage
A group of students studied the reactivity
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents