The reaction below was performed under thermodynamic conditions to yield Compound 1 and Compound 2 as a mixture of structural isomers. If Compound 1 is more stable than Compound 2, what can be inferred about the relative amounts of Compound 1 to Compound 2 in the mixture? 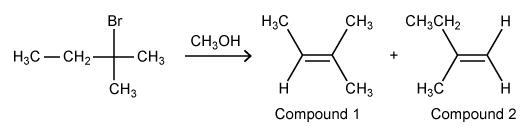
A) There is a greater amount of Compound 1 in the mixture.
B) There is a greater amount of Compound 2 in the mixture.
C) There are equal amounts of Compound 1 and Compound 2 in the mixture.
D) Nothing can be inferred about the relative amounts of the compounds in the mixture.
Correct Answer:
Verified
Q97: Which separation technique is optimal for purification
Q98: Terpenes are made up of two or
Q99: Passage
Electrospray ionization mass spectrometry (ESI-MS) employs a
Q100: What is the product of the reaction
Q101: Hydroxyl substituents in alcohols are poor leaving
Q103: Passage
Students used three classification tests to study
Q104: The amino acids Val, Leu, and Ile
Q105: What functional group forms in an acid-catalyzed
Q106: Passage
Students used three classification tests to study
Q107: A wax is made up of a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents