Passage The Protozoan Euglena Gracilis Expresses Glycosyltransferases (Enzymes That Add Carbohydrates
Passage
The protozoan Euglena gracilis expresses glycosyltransferases (enzymes that add carbohydrates to molecules) and glycosidases (enzymes that remove carbohydrates) within its membrane. These enzymes were previously studied using radiolabeled sugars. To avoid the use of radioactive materials, fluorescence-based assays were investigated. Derivatives of the fluorescent molecule coumarin were attached to carbohydrates (Figure 1) for detection in fluorescence assays.Compound 1, a carbohydrate derivative with hydroxyl groups protected by benzoate (Bz) , reacted with Compound 2 to form Compound 3. 13C NMR confirmed the formation of Compound 3 by observing a distinct C-H coupling at the anomeric carbon. Hydroxyl deprotection and addition of Compound 4 (a coumarin analogue) gave Compound 5, a fluorescent coumarin derivative. Compound 6, another coumarin derivative, was made following a similar sequence except an additional sugar was attached before the addition of the coumarin group.
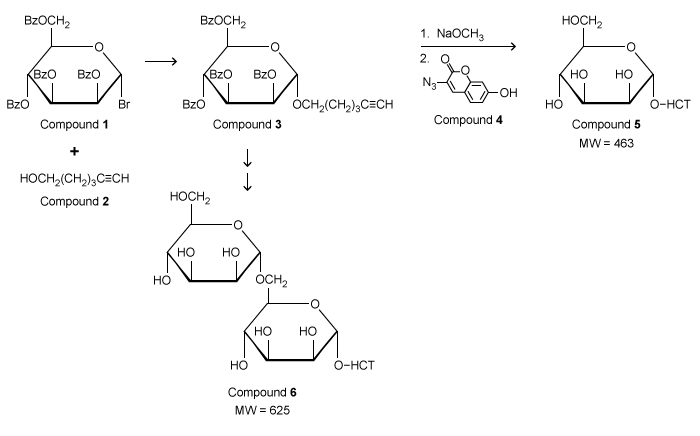 Figure 1 Synthesis of fluorescent coumarin derivatives (Note: HCT denotes the fluorescent butyl hydroxycoumarin triazole group.) Small vesicles called microsomes were isolated from Euglena gracilis membranes, and microsomal glycosyltransferase activities were investigated using a hexose donor substrate and either Compound 5 (Figure 2) or Compound 6 (Figure 3) as the acceptor substrate. Thin-layer chromatography (TLC) analysis indicated the formation of a new product in each assay. These products were purified and characterized by liquid chromatography-mass spectrometry (LC-MS) . The molecular ion for each product was subjected to another round of MS, in which individual hexose units (MW) = 162) were cleaved from the coumarin derivatives that were formed during the assay. The MW of hydroxycoumarin triazole (HCT) is 301.
Figure 1 Synthesis of fluorescent coumarin derivatives (Note: HCT denotes the fluorescent butyl hydroxycoumarin triazole group.) Small vesicles called microsomes were isolated from Euglena gracilis membranes, and microsomal glycosyltransferase activities were investigated using a hexose donor substrate and either Compound 5 (Figure 2) or Compound 6 (Figure 3) as the acceptor substrate. Thin-layer chromatography (TLC) analysis indicated the formation of a new product in each assay. These products were purified and characterized by liquid chromatography-mass spectrometry (LC-MS) . The molecular ion for each product was subjected to another round of MS, in which individual hexose units (MW) = 162) were cleaved from the coumarin derivatives that were formed during the assay. The MW of hydroxycoumarin triazole (HCT) is 301.
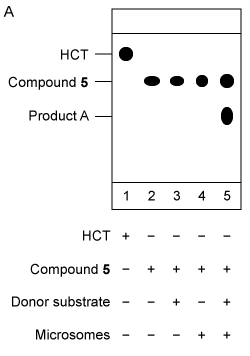
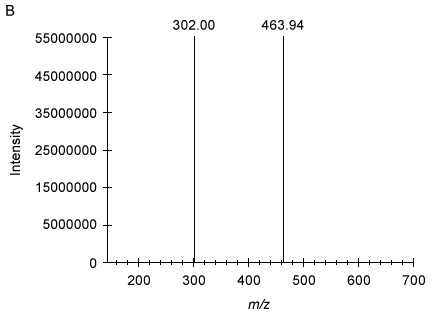 Figure 2 Analysis of Compound 5 as the acceptor substrate: (A) TLC analysis of fluorescent products; (B) Mass spectrum of product A fragmented molecular ion peak
Figure 2 Analysis of Compound 5 as the acceptor substrate: (A) TLC analysis of fluorescent products; (B) Mass spectrum of product A fragmented molecular ion peak
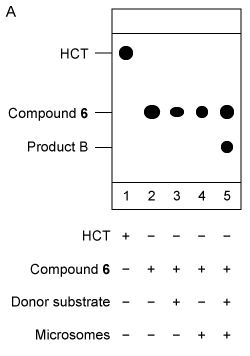
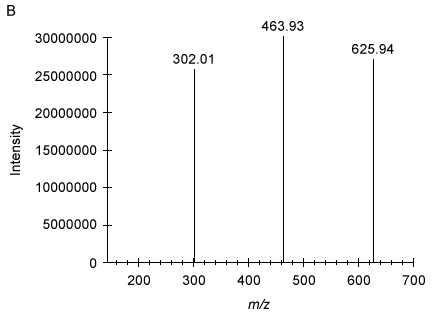 Figure 3 Analysis of Compound 6 as the acceptor substrate: (A) TLC analysis of fluorescent products; (B) Mass spectrum of product B fragmented molecular ion peak
Figure 3 Analysis of Compound 6 as the acceptor substrate: (A) TLC analysis of fluorescent products; (B) Mass spectrum of product B fragmented molecular ion peak
Adapted from: I. M. Ivanova et al., "Fluorescent mannosides serve as acceptor substrates for glycosyltransferase and sugar-1-phosphate transferase activities in Euglena gracilis membranes." Carbohydrate Research. © 2017 Elsevier.
-Consider the cyclic form of the sugar from which Compound 1 is derived. Can this sugar be classified as a pyranose and a hemiacetal?
A) Yes; pyranoses are six-membered rings and hemiacetals are formed from aldoses.
B) Yes; pyranoses are five-membered rings and hemiacetals are formed from ketoses.
C) No; pyranoses are five-membered rings and hemiacetals are formed from aldoses.
D) No; pyranoses are six-membered rings and hemiacetals are formed from ketoses.
Correct Answer:
Verified
Q154: The compound shown below has selected carbons
Q155: Passage
The N-terminus of an amino acid must
Q156: Four alkyl halides: bromomethane, 2-bromobutane, 2-bromo-2-methylpropane, and
Q157: Passage
Malaria is caused by the parasite Plasmodium
Q158: Passage
Malaria is caused by the parasite Plasmodium
Q160: A 100-mL aqueous HCl solution was made
Q161: The infrared spectrum shows the frequencies of
Q162: Which term can be used to classify
Q163: Which of the following statements correctly describes
Q164: Passage
Students perform a synthesis of a carboxylic
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents