Passage
Malaria is caused by the parasite Plasmodium falciparum and is transmitted through a female mosquito bite. Although there are antimalarial treatments available, P. falciparum has become resistant to many of these drugs. P. falciparum cells contain a respiratory organelle called the apicoplast that is necessary for the parasite's survival but is not found in humans. Therefore, a new drug that targets this organelle could be useful in the treatment of malaria.A portion of the apicoplast protein ferredoxin (PfFd) is shown in Figure 1, with certain amino acid residues labeled. In the apicoplast, ferredoxin NADP+ reductase (PfFNR) interacts electrostatically with PfFd and catalyzes an electron transfer reaction. A compound that selectively inhibits this interaction could be a beneficial antimalarial agent.
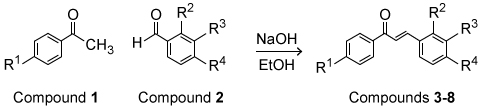
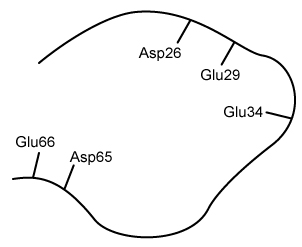 Figure 1 Structure of a portion of PfFdChalcone (Compound 3) is a compound that interacts with PfFd and exhibits antimalarial properties. A series of chalcone derivatives (Compounds 4-8) were synthesized via the aldol condensation shown in Scheme 1, where nucleophilic addition of Compound 1 to Compound 2 is followed by elimination to yield α,β-unsaturated carbonyl molecules, Compounds 3-8.
Figure 1 Structure of a portion of PfFdChalcone (Compound 3) is a compound that interacts with PfFd and exhibits antimalarial properties. A series of chalcone derivatives (Compounds 4-8) were synthesized via the aldol condensation shown in Scheme 1, where nucleophilic addition of Compound 1 to Compound 2 is followed by elimination to yield α,β-unsaturated carbonyl molecules, Compounds 3-8.
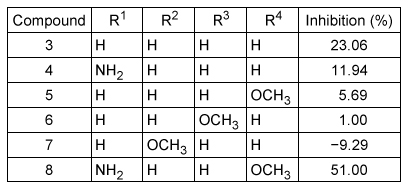
 Scheme 1An inhibition assay was performed to compare chalcone (Compound 3) and its derivatives by determining the extent to which these compounds inhibited electron transfer between PfFNR and PfFd (Table 1) .Table 1 Inhibition of electron transfer assay results
Scheme 1An inhibition assay was performed to compare chalcone (Compound 3) and its derivatives by determining the extent to which these compounds inhibited electron transfer between PfFNR and PfFd (Table 1) .Table 1 Inhibition of electron transfer assay results
 Adapted from H. Suwito et al. "Design and synthesis of chalcone derivatives as inhibitors of the ferredoxin - ferredoxin-NADP+ reductase interaction of Plasmodium falciparum: pursuing new antimalarial agents." Molecules. ©2014 MDPI.
Adapted from H. Suwito et al. "Design and synthesis of chalcone derivatives as inhibitors of the ferredoxin - ferredoxin-NADP+ reductase interaction of Plasmodium falciparum: pursuing new antimalarial agents." Molecules. ©2014 MDPI.
-Based on the data in Table 1, what effect do the chalcone derivative substituents have on the percent inhibition relative to chalcone?
A) An NH2 substituent alone has a positive impact on percent inhibition, but an OCH3 substituent alone has no impact.
B) An NH2 substituent alone has a positive impact on percent inhibition, but an OCH3 substituent alone has a negative impact.
C) Chalcone derivatives with NH2 and OCH3 substituents impact the percent inhibition and are both necessary to increase the percent inhibition.
D) Chalcone derivatives with NH2 and OCH3 substituents impact the percent inhibition, but only one is necessary to increase the percent inhibition.
Correct Answer:
Verified
Q152: Passage
The N-terminus of an amino acid must
Q153: Passage
The N-terminus of an amino acid must
Q154: The compound shown below has selected carbons
Q155: Passage
The N-terminus of an amino acid must
Q156: Four alkyl halides: bromomethane, 2-bromobutane, 2-bromo-2-methylpropane, and
Q158: Passage
Malaria is caused by the parasite Plasmodium
Q159: Passage
The protozoan Euglena gracilis expresses glycosyltransferases (enzymes
Q160: A 100-mL aqueous HCl solution was made
Q161: The infrared spectrum shows the frequencies of
Q162: Which term can be used to classify
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents