With respect to the Maxwell-Boltzmann probability distribution function of molecular speeds, which of the following is true?
A)
B)
C) 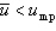
D)
E) A and D
Correct Answer:
Verified
Q23: Comparing the van der Waals b constant
Q24: At the same temperature and pressure, comparing
Q25: Which of the following in gases is
Q26: If the compressibility factor for a real
Q27: Helium effuses through a small opening at
Q28: Air is 78.1% N2 gas. This is
Q29: Which has more average kinetic energy per
Q30: A 5 L container is held at
Q31: Based on the van der Waals
Q32: The compressibility factor (z) for an ideal
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents