The following questions refer to the phase diagram below. Gas, liquid, and solid phases are all present, but not labeled. 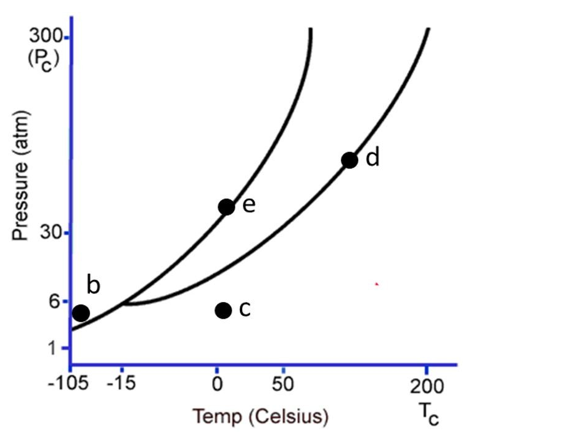
-At the temperature and pressure of point d, which statement below is true?
A) The substance will sublime.
B) There will be an equilibrium between the solid phase and the gaseous phase.
C) Vaporization and deposition will take place simultaneously.
D) Condensation and evaporation will take place simultaneously.
E) The substance will be a supercritical fluid.
Correct Answer:
Verified
Q53: Dioxane, C4H8O2, has an enthalpy of fusion
Q54: A substance, B, has a normal boiling
Q55: Evaporation
A)is an endothermic process.
B)is an exothermic process.
C)involves
Q56: An isobar is a line of constant
Q57: The triple point of a substance is
Q59: The following questions refer to the phase
Q60: The following questions refer to the phase
Q61: The following questions refer to the phase
Q62: The following questions refer to the phase
Q63: The following questions refer to the phase
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents