The following questions refer to the phase diagram below. Gas, liquid, and solid phases are all present, but not labeled. 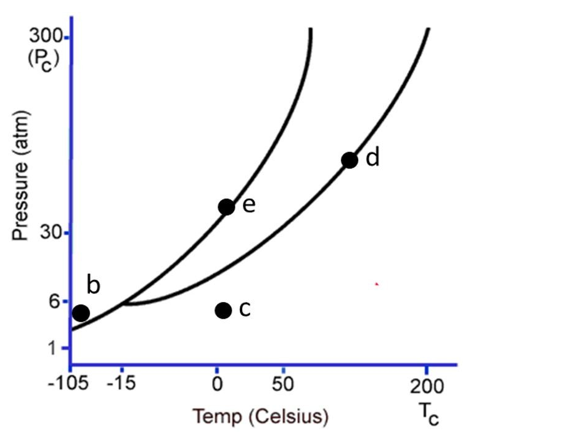
-Starting at the temperature and pressure of b, if the temperature is increased at constant pressure, ultimately
A) the substance will sublime.
B) the substance will undergo fusion.
C) the substance will undergo deposition.
D) the substance will freeze.
E) the substance will be a supercritical fluid.
Correct Answer:
Verified
Q55: Evaporation
A)is an endothermic process.
B)is an exothermic process.
C)involves
Q56: An isobar is a line of constant
Q57: The triple point of a substance is
Q58: The following questions refer to the phase
Q59: The following questions refer to the phase
Q61: The following questions refer to the phase
Q62: The following questions refer to the phase
Q63: The following questions refer to the phase
Q64: The following questions refer to the phase
Q65: The following questions refer to the phase
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents