Short Answer
Consider the equilibrium C(s)+ H2O(g) 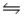
CO(g)+ H2(g), H = 2296 J What will happen to the concentration of carbon monoxide if the temperature of this system is raised?
Correct Answer:
Verified
Related Questions
Q95: Consider the chemical reaction 2NH3(g) 
Q96: Consider the equilibrium equation C(s)+ H2O(g)
Q97: Consider the following equilibrium,
4NH3(g)+ 3O2(g) 
Q98: The Kc for the reaction CO2(g)+ H2(g)
Q99: Hydrogen iodide decomposes according to the equation:
2HI(g)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents