The Kc for the reaction CO2(g)+ H2(g) 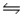
H2O(g)+ CO(g)is 1.6 at about 990ºC.Calculate the number of moles of carbon dioxide in the final equilibrium system obtained by initially adding 1.00 mol of H2, 2.00 mol of CO2, 0.750 mol of H2O, and 1.00 mol of CO to a 5.00 L reactor at 990ºC.
Correct Answer:
Verified
Q93: Consider the equilibrium C(s)+ H2O(g)
Q94: Hydrogen iodide decomposes according to the equation:
2HI(g)
Q95: Consider the chemical reaction 2NH3(g) 
Q96: Consider the equilibrium equation C(s)+ H2O(g)
Q97: Consider the following equilibrium,
4NH3(g)+ 3O2(g) 
Q99: Hydrogen iodide decomposes according to the equation:
2HI(g)
Q100: Consider the equilibrium C(s)+ H2O(g)
Q101: When the reaction 2O3(g) Q102: The equilibrium between carbon dioxide gas and Q103: The Kc for the reaction CO2(g)+ H2(g)![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents