The equilibrium constant for the gas phase reaction N2 (g) + 3H2 (g) 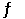 2NH3 (g)
2NH3 (g)
Is Keq = 4.34 × 10-3 at 300°C. At equilibrium, __________.
A) products predominate
B) reactants predominate
C) roughly equal amounts of products and reactants are present
D) only products are present
E) only reactants are present
Correct Answer:
Verified
Q28: Of the following equilibria, only _ will
Q29: How is the reaction quotient used to
Q30: At 400 K, the equilibrium constant for
Q31: The expression for Kp for the reaction
Q32: The equilibrium constant for the gas phase
Q34: The equilibrium-constant expression for the reaction Ti
Q35: Which of the following expressions is the
Q36: The equilibrium constant for the gas phase
Q37: Which of the following expressions is the
Q38: The reaction below is exothermic: 2SO2 (g)+
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents