Of the following equilibria, only __________ will shift to the left in response to a decrease in volume.
A) H2 (g) + Cl2 (g) 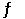 2 HCl (g)
2 HCl (g)
B) 2 SO3 (g) 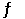 2 SO2 (g) + O2 (g)
2 SO2 (g) + O2 (g)
C) N2 (g) + 3 H2 (g) 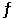 2 NH3 (g)
2 NH3 (g)
D) 4 Fe (s) + 3 O2 (g) 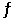 2 Fe2O3 (s)
2 Fe2O3 (s)
E) 2HI (g) 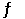 H2 (g) + I2 (g)
H2 (g) + I2 (g)
Correct Answer:
Verified
Q23: Consider the following equilibrium. 2SO2 (g)+ O2
Q24: Of the following equilibria, only _ will
Q25: Which of the following expressions is the
Q26: For the endothermic reaction CaCO3 (s)
Q27: In which of the following reactions would
Q29: How is the reaction quotient used to
Q30: At 400 K, the equilibrium constant for
Q31: The expression for Kp for the reaction
Q32: The equilibrium constant for the gas phase
Q33: The equilibrium constant for the gas phase
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents