Shown below is a concentration vs.time plot for the reaction A ⇌ 2B.For this reaction the value of the equilibrium constant is 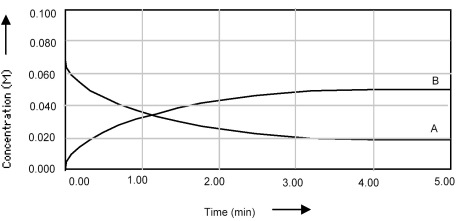
A) Kc < 1.
B) Kc = 0.
C) Kc = 1.
D) Kc > 1.
Correct Answer:
Verified
Q78: A catalyst increases the overall rate of
Q79: At 25°C,a certain first order reaction has
Q80: The following pictures represent the equilibrium state
Q81: Picture (1)represents the equilibrium mixture for the
Q82: The following pictures represent mixtures of cis-C2H2X2
Q84: The following pictures represent mixtures of A2B4
Q85: The reaction A2 + B2 ⇌ 2AB
Q86: The reaction A2 + B2 ⇌ 2AB
Q87: Shown below is a concentration vs.time plot
Q88: Shown below is a concentration vs.time plot
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents