The concentration-time dependence is shown below for two first-order reactions is: 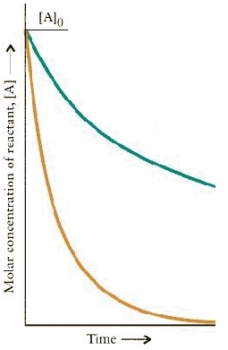 Which reaction has the larger rate constant?
Which reaction has the larger rate constant?
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q3: For a given first-order reaction, after 2.00
Q15: The concentration-time curves for two sets of
Q16: Given: 2A(g)+ B(g)
Q17: A first-order reaction has a rate constant
Q18: The concentration-time curves for two sets of
Q24: A nonsteroidal anti-inflammatory drug is metabolized with
Q33: For a second-order reaction, a straight line
Q35: Given: A
Q38: A first-order reaction has a half-life of
Q58: For the reaction cyclobutane(g)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents