The concentration-time curves for two sets of reactions,A/B and C/D,are: 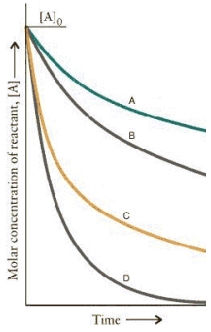 Which set of reactions has the largest rate constant?
Which set of reactions has the largest rate constant?
Correct Answer:
Verified
Q3: For a given first-order reaction, after 2.00
Q10: It is important to distinguish between the
Q15: The concentration-time curves for two sets of
Q16: Given: 2A(g)+ B(g)
Q17: A first-order reaction has a rate constant
Q19: The rate of formation of oxygen
Q20: The concentration-time dependence is shown below for
Q24: A nonsteroidal anti-inflammatory drug is metabolized with
Q33: For a second-order reaction, a straight line
Q35: Given: A
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents